Beckingham Group Research
Membrane Materials and Characterization
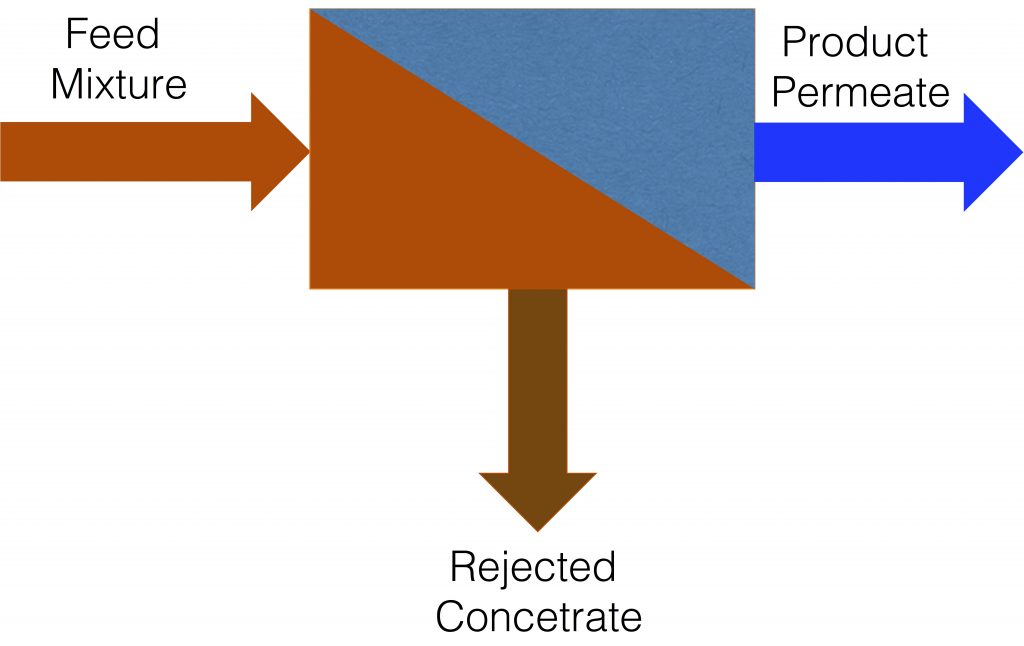
One fifth of the world’s population is believed to live in regions of the world that lack sufficient access water. It is predicted that within the next 10 years two-thirds of the world’s population will be living in a water stressed area. Membrane-based processes (in particular polymer-based membranes) are currently a leading technology for desalination and going forward there is increasing need to purify other saline and contaminated water sources to satisfy growing water needs. Thus, improved polymer membranes are needed for economical and sustainable water purification. Additionally, these processes have great potential for improving the sustainability of other industrial processes and separations. Our research seeks to understand how molecules and ions permeate through and interact with and within polymer membranes.
Polymer Synthesis
In order to perform nearly all of our work the synthesis of well-defined polymers with controlled macromolecular structure is required. We typically synthesize new polymers using so-called “living” polymerization chemistries where due to a lack of spontaneous termination we can finely control the resulting molecular weight and molecular architecture through precise initiation of the polymerization and sequential addition of monomeric species. In order to achieve this control over the polymerization the rigorous purification of solvents and monomers is required as is a highly controlled polymerization environment which we achieve through glove box and schlenk line techniques.
Living Polymerization
Using anionic and other living polymerizations, we can synthesize a wide variety of homopolymer and block copolymer materials with narrow molecular weight distributions (<1.1) and well-defined structures. Anionic polymerization proceeds by a chain growth mechanisms where an initiator (such as organolithiums or alkali metals) reacts with a monomer to form an active growing chain. Since the growing chains in unreactive to each other and not susceptible to other termination reactions the active species is “living.” In order to perform rigorous removal of reactive contaminants such as oxygen and water and to handle the highly pyrophoric initiators, we utilize a nitrogen environment and high-vacuum Schlenk lines as shown below. As all chains were initiated at approximately the same time and will react with monomer and grow at the same rate the resulting polymers will have approximately the same length. Once the desired conversion is achieved, the active chain ends a terminating species is added to kill the living chain. Through complete conversion and sequential monomer addition block copolymers can be synthesized and by judicious choice of terminating species chains ends can be tailored with specific functional groups for subsequent modifications.
Polymer Additive Manufacturing
Additive manufacturing (also known as 3D printing) of polymer materials has existed as a technology for approximately 30 years but has recently seen a dramatic increase in both attention and use in industry, among the general population and in academia. This expansion of interest has been driven by decreasing costs of 3D printing equipment, improvements in print quality and overall improvement in ease-of-use through software improvements. There are two dominant types of 3D printing techniques for polymer materials: Fused Deposition Modeling (FDM) and stereolithography (SLA). These two 3D printing processes are shown schematically in Figure 1.[1] FDM utilizes a filament or wire of polymer material that is extruded through a hot nozzle in a layer-by-layer fashion to print an object. FDM has most prominently found application for rapid prototyping. SLA also builds an object layer-by-layer but does so by using photopolymerization. During SLA a beam of light is focused to a spot within a bath of liquid resin where it causes a reaction that forms the solid polymer. By controlling the light position and resin chemistry desired objects are printed from this liquid resin bath into the final solid object.
Recent advances in these two 3D printing technologies notwithstanding, there remain many opportunities for progress and especially so in the area of materials design. Currently, polymeric 3D printing is rather limited in the types of polymer materials and thereby material properties of finished objects that can be produced. Additionally, many of the polymer material options for 3D printing are not intrinsically recyclable requiring disposal if they suffer any damage. Here, we aim to leverage experience and expertise in polymer chemistry and self-healing composite materials to improve the quality and lifetime of 3D printed polymeric objects.
Conjugated Polymers: Polythiophenes
The performance of devices based on semiconducting polymers is critically linked to the underlying polymer properties and processing pathway such that control over polymer solubility, crystallinity, thermal conductivity, absorption efficiency, band gap, and charge carrier mobility are paramount. Our research on conjugated polymers is focused on understanding underlying structure-property relationships in this class of materials in order to inform the design of next-generational semiconducting polymers. Development of poly(3-alkylthiophene)s (P3AT)s over the last decade has been primarily motivated by their improved solubility over un-substituted polythiophene while retaining good semiconducting properties such as low optical band gap, relatively high mobilities. Thus, via the attachment of groups of varying functionalities (such as various alkyl, alkoxy, nitro etc.) the solubility, solid-state structure and optoelectronic properties have been investigated. Currently, a variety of regioregular P3ATs have been examined for application as semiconductors but there remains many opportunities for improvement in properties and understanding of the underlying structure-property relationships.